Is ‘light-speed’ vaccine development still too slow?
The aim of this game is twofold. The first goal is to get labs match-fit for rapid vaccine development upon the emergence of Disease X. The second is to build a library of “prototype” vaccines for each of the 25 virus families that pose a pandemic threat to humans.
“My team are facing a lot of pressure, they’re excited about it and nervous,” Chappell says of the challenge. While CEPI’s eventual goal is to get labs cracking on a 100-day timescale, Chappell’s team is aiming for the slightly more achievable, but still daunting, deadline of 150 days.
Until Pfizer’s 337-day COVID-19 vaccine, the fastest development timeline for a vaccine was four years.Credit: AP
The fastest vaccine ever made was one of Pfizer’s early COVID-19 jabs, which took 337 days between identification of the disease and approval of the vaccine. And that had the eyes, funding and support of the entire world. Before the COVID pandemic, the fastest vaccine was for mumps; it took four years.
“So this is light-speed for this type of work,” Chappell says of the 150-day goal.
But even that may be too slow to meet CEPI’s goal of ending the existential threat of pandemics. According to Professor Sir Jeremy Farrar, former chief scientist of the World Health Organisation and one of the authors of an influential paper that led to CEPI’s formation in 2017, to truly end the threat of pandemics we’d need to cut the vaccine-making window to a mere 30 days. A 100-day vaccine wouldn’t have stopped COVID-19 going global.
But that’s not to say a three-month vaccine couldn’t have enormous benefits. A recent study in The Lancet used modelling to estimate how the pandemic would’ve played out if the COVID-19 vaccine had been made and released within 100 days of the virus’s genome being identified.
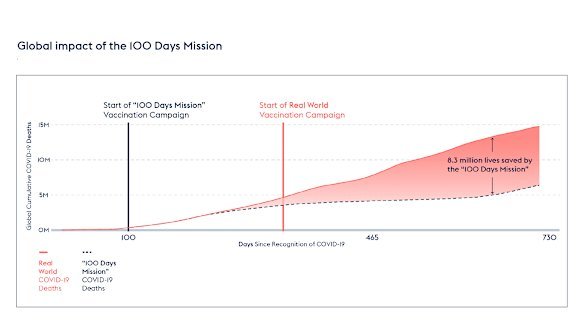
Modelling published in The Lancet found 8.3 million lives may have been saved – mostly in low-income countries – if a COVID vaccine had been rolled out within 100 days.Credit: CEPI
Their conclusion: it would have saved 8.33 million people from dying of COVID by the end of 2021 – 57 per cent of the global deaths due to the virus to that date – and prevented $14.35 trillion of economic havoc.
Clamping down on novel viruses
Chappell is one of the inventors of “molecular clamp” vaccine technology, which he’s using to pursue a vaccine for Chapare virus. The technique works by borrowing a non-infectious protein from a virus to grab onto a lab-made spike protein.
“Viral families such as coronaviruses, influenza viruses, ebola, all have these spike proteins on their surface. But they’re naturally very hard to produce in a lab or manufacturing setting,” Chappell says.
The molecular clamp, shown in red, stabilising a COVID-19 spike protein to be presented to the body’s immune system.Credit: Dr Dan Watterson, The University of Queensland
“What the molecular clamp does is allow us to produce those proteins at high quantities and ensure that they’re locked into the optimal conformation so that they produce a strong neutralising immune response.”
The spike protein triggers the body to produce antibodies against the virus, building resistance to infection. The clamp is key because spike proteins quickly deform in the body; they jackknife when they stab into a host cell, fusing with the membrane and allowing the virus to invade.
Once this fusion occurs and spikes deform, they trigger a much weaker immune response. But when fused to a molecular clamp, the spike protein is held tightly in shape and doesn’t deform, so it triggers the robust immune response you want from a vaccine.
The actual clamp stays the same between each new vaccine; Chappell and his team just need the sequence of the spike protein for whatever virus they’re trying to immunise against so they can grow it in the lab and stick it to the clamp. That’s what makes the method well suited to rapid vaccine development.
But doing it all in 150 days is unprecedented.
“You need to iron out all the issues, all the kinks, everything that can go wrong,” Chappell said. “It really is a moonshot goal to try to have a vaccine that’s safe, effective and of the right quality to be used in humans within that sort of time frame.”
That’s a lesson they learnt in 2020.
Proteins from different viruses attached to the molecular clamp.Credit: The University of Queensland
Fixing false HIV readings
The team were racing to create a COVID-19 vaccine with their molecular clamp tech in 2020, and had progressed to human trials, but then had to call off the project after their vaccine triggered false-positive HIV results in some patients.
There was never any actual chance of HIV infection, but the iteration of the clamp they were using had been constructed out of proteins borrowed from HIV, hence the false-positives. The devastated scientists shelved the project.
They have spent the past few years rebuilding the clamp using a different viral protein, derived from an Icelandic sheep virus that has never infected humans.
Loading
Now they’re brewing up a 50-litre batch of a version of the clamp fused to a Chapare virus spike protein – enough to theoretically immunise millions of people.
The goal is to finalise the vaccine, test it in mice and provide a dossier of safety information to an independent board of reviewers for analysis before the 150 days are up in July.
“We’re essentially asking the question: if this was an actual emergency, would you give us permission to put this into humans?” Chappell says.
“We’re looking forward to getting that feedback – because that’s what we’re trying to look at, the transparency of making vaccines fast.”
Enjoyed Examine, our free weekly newsletter covering science with a sceptical, evidence-based eye? Sign up to get the whole newsletter in your inbox.
Read the full article here